Radix Institute
Neuroscience and Radix®Rhythm of Breathing Affects Memory and Fear
Summary: A new study reports the rhythm of your breathing can influence neural activity that enhances memory recall and emotional judgement.
Source: Northwestern University.
Breathing is not just for oxygen; it’s now linked to brain function and behavior.
Northwestern Medicine scientists have discovered for the first time that the rhythm of breathing creates electrical activity in the human brain that enhances emotional judgments and memory recall.
These effects on behavior depend critically on whether you inhale or exhale and whether you breathe through the nose or mouth.
In the study, individuals were able to identify a fearful face more quickly if they encountered the face when breathing in compared to breathing out. Individuals also were more likely to remember an object if they encountered it on the inhaled breath than the exhaled one. The effect disappeared if breathing was through the mouth.
“One of the major findings in this study is that there is a dramatic difference in brain activity in the amygdala and hippocampus during inhalation compared with exhalation,” said lead author Christina Zelano, assistant professor of neurology at Northwestern University Feinberg School of Medicine. “When you breathe in, we discovered you are stimulating neurons in the olfactory cortex, amygdala and hippocampus, all across the limbic system.”
The study was published Dec. 6 in the Journal of Neuroscience.
The senior author is Jay Gottfried, professor of neurology at Feinberg.
Northwestern scientists first discovered these differences in brain activity while studying seven patients with epilepsy who were scheduled for brain surgery. A week prior to surgery, a surgeon implanted electrodes into the patients’ brains in order to identify the origin of their seizures. This allowed scientists to acquire electro-physiological data directly from their brains. The recorded electrical signals showed brain activity fluctuated with breathing. The activity occurs in brain areas where emotions, memory and smells are processed.
This discovery led scientists to ask whether cognitive functions typically associated with these brain areas — in particular fear processing and memory — could also be affected by breathing.
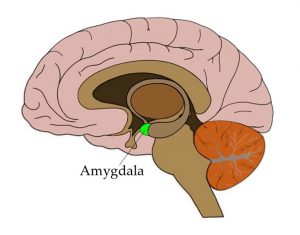
The amygdala is strongly linked to emotional processing, in particular fear-related emotions. So scientists asked about 60 subjects to make
rapid decisions on emotional expressions in the lab environment while recording their breathing. Presented with pictures of faces showing expressions of either fear or surprise, the subjects had to indicate, as quickly as they could, which emotion each face was expressing. NeuroscienceNews.com image
is for illustrtive purposes only.
The amygdala is strongly linked to emotional processing, in particular fear-related emotions. So scientists asked about 60 subjects to make rapid decisions on emotional expressions in the lab environment while recording their breathing. Presented with pictures of faces showing expressions of either fear or surprise, the subjects had to indicate, as quickly as they could, which emotion each face was expressing.
When faces were encountered during inhalation, subjects recognized them as fearful more quickly than when faces were encountered during exhalation. This was not true for faces expressing surprise. These effects diminished when subjects performed the same task while breathing through their mouths. Thus the effect was specific to fearful stimuli during nasal breathing only.
In an experiment aimed at assessing memory function — tied to the hippocampus — the same subjects were shown pictures of objects on a computer screen and told to remember them. Later, they were asked to recall those objects. Researchers found that recall was better if the images were encountered during inhalation.
The findings imply that rapid breathing may confer an advantage when someone is in a dangerous situation, Zelano said.
“If you are in a panic state, your breathing rhythm becomes faster,” Zelano said. “As a result you’ll spend proportionally more time inhaling than when in a calm state. Thus, our body’s innate response to fear with faster breathing could have a positive impact on brain function and result in faster response times to dangerous stimuli in the environment.”
Another potential insight of the research is on the basic mechanisms of meditation or focused breathing. “When you inhale, you are in a sense synchronizing brain oscillations across the limbic network,” Zelano noted.
Other Northwestern authors include Heidi Jiang, Guangyu Zhou, Nikita Arora, Dr. Stephan Schuele and Dr. Joshua Rosenow.
Funding: The study was supported by grants R00DC012803, R21DC012014 and R01DC013243 from the National Institute on Deafness and Communication Disorders of the National Institutes of Health.
Source: Marla Paul – Northwestern University
Image Source: NeuroscienceNews.com image is in the public domain.
Video Source: The video is credited to NorthwesternU.
Original Research: Abstract for “Nasal Respiration Entrains Human Limbic Oscillations and Modulates Cognitive Function” by Christina Zelano, Heidi Jiang, Guangyu Zhou, Nikita Arora, Stephan Schuele, Joshua Rosenow and Jay A. Gottfried in Journal of Neuroscience. Published online December 7 2016 doi:10.1523/JNEUROSCI.2586-16.2016
<http://neurosciencenews.com/memory-fear-breathing-5699/>.
Abstract
Nasal Respiration Entrains Human Limbic Oscillations and Modulates Cognitive Function
The need to breathe links the mammalian olfactory system inextricably to the respiratory rhythms that draw air through the nose. In rodents and other small animals, slow oscillations of local field potential activity are driven at the rate of breathing (∼2–12 Hz) in olfactory bulb and cortex, and faster oscillatory bursts are coupled to specific phases of the respiratory cycle. These dynamic rhythms are thought to regulate cortical excitability and coordinate network interactions, helping to shape olfactory coding, memory, and behavior. However, while respiratory oscillations are a ubiquitous hallmark of olfactory system function in animals, direct evidence for such patterns is lacking in humans. In this study, we acquired intracranial EEG data from rare patients (Ps) with medically refractory epilepsy, enabling us to test the hypothesis that cortical oscillatory activity would be entrained to the human respiratory cycle, albeit at the much slower rhythm of ∼0.16–0.33 Hz. Our results reveal that natural breathing synchronizes electrical activity in human piriform (olfactory) cortex, as well as in limbic-related brain areas, including amygdala and hippocampus. Notably, oscillatory power peaked during inspiration and dissipated when breathing was diverted from nose to mouth. Parallel behavioral experiments showed that breathing phase enhances fear discrimination and memory retrieval. Our findings provide a unique framework for understanding the pivotal role of nasal breathing in coordinating neuronal oscillations to support stimulus processing and behavior.
SIGNIFICANCE STATEMENT Animal studies have long shown that olfactory oscillatory activity emerges in line with the natural rhythm of breathing, even in the absence of an odor stimulus. Whether the breathing cycle induces cortical oscillations in the human brain is poorly understood. In this study, we collected intracranial EEG data from rare patients with medically intractable epilepsy, and found evidence for respiratory entrainment of local field potential activity in human piriform cortex, amygdala, and hippocampus. These effects diminished when breathing was diverted to the mouth, highlighting the importance of nasal airflow for generating respiratory oscillations. Finally, behavioral data in healthy subjects suggest that breathing phase systematically influences cognitive tasks related to amygdala and hippocampal functions.
“Nasal Respiration Entrains Human Limbic Oscillations and Modulates Cognitive Function” by Christina Zelano, Heidi Jiang, Guangyu Zhou, Nikita Arora, Stephan Schuele, Joshua Rosenow and Jay A. Gottfried in Journal of Neuroscience. Published online December 7 2016 doi:10.1523/JNEUROSCI.2586-16.2016
Pioneers of Neuroscience

The polyvagal theory: New insights into adaptive reactions of the autonomic nervous system
In 1992, I proposed that an estimate of vagal tone, derived from measuring respiratory sinus arrhythmia, could be used in clinical medicine as an index of stress vulnerability. Rather than using the descriptive measures of heart rate variability (ie, beat-to-beat variability) frequently used in obstetrics and pediatrics, the paper emphasized that respiratory sinus arrhythmia has a neural origin and represents the tonic functional outflow from the vagus to the heart (ie, cardiac vagal tone). Thus, it was proposed that respiratory sinus arrhythmia would provide a more sensitive index of health status than a more global measure of beat-to-beat heart rate variability reflecting undetermined neural and nonneural mechanisms.
The paper presented a quantitative approach that applied time-series analyses to extract the amplitude of respiratory sinus arrhythmia as a more accurate index of vagal activity. The article provided data demonstrating that healthy full-term infants had respiratory sinus arrhythmia of significantly greater amplitude than did preterm infants. This idea of using heart rate patterns to index vagal activity was not new, having been reported as early as 1910 by Hering.5 Moreover, contemporary studies have reliably reported that vagal blockade via atropine depresses respiratory sinus arrhythmia in mammals.6,7
THE POLYVAGAL THEORY: THREE PHYLOGENETIC RESPONSE SYSTEMS
Investigation of the phylogeny of the vertebrate autonomic nervous system provides an answer to the vagal paradox. Research in comparative neuroanatomy and neurophysiology has identified two branches of the vagus, with each branch supporting different adaptive functions and behavioral strategies. The vagal output to the heart from one branch is manifested in respiratory sinus arrhythmia, and the output from the other branch is manifested in bradycardia and possibly the slower rhythms in heart rate variability. Although the slower rhythms have been assumed to have a sympathetic influence, they are blocked by atropine.7
The polyvagal theory7,11–15 articulates how each of three phylogenetic stages in the development of the vertebrate autonomic nervous system is associated with a distinct autonomic subsystem that is retained and expressed in mammals. These autonomic subsystems are phylogenetically ordered and behaviorally linked to social communication (eg, facial expression, vocalization, listening), mobilization (eg, fight–flight behaviors), and immobilization (eg, feigning death, vasovagal syncope, and behavioral shutdown).
The social communication system (ie, social engagement system; see below) involves the myelinated vagus, which serves to foster calm behavioral states by inhibiting sympathetic influences to the heart and dampening the hypothalamic-pituitary-adrenal (HPA) axis.16 The mobilization system is dependent on the functioning of the sympathetic nervous system. The most phylo-genetically primitive component, the immobilization system, is dependent on the unmyelinated vagus, which is shared with most vertebrates. With increased neural complexity resulting from phylogenetic development, the organism’s behavioral and affective repertoire is enriched. The three circuits can be conceptualized as dynamic, providing adaptive responses to safe, dangerous, and life-threatening events and contexts.
Only mammals have a myelinated vagus. Unlike the unmyelinated vagus, originating in the dorsal motor nucleus of the vagus with pre- and postganglionic muscarinic receptors, the mammalian myelinated vagus originates in the nucleus ambiguus and has preganglionic nicotinic receptors and postganglionic muscarinic receptors. The unmyelinated vagus is shared with other vertebrates, including reptiles, amphibians, teleosts, and elasmobranchs.
Bodily state is regulated in an efficient manner to promote growth and restoration
Investigation of the phylogeny of regulation of the vertebrate heart11,12,19,20 has led to extraction of four principles that provide a basis for testing of hypotheses relating specifi c neural mechanisms to social engagement, fight–flight, and death-feigning behaviors:
-
There is a phylogenetic shift in the regulation of the heart from endocrine communication to unmyelinated nerves and finally to myelinated nerves.
-
There is a development of opposing neural mechanisms of excitation and inhibition to provide rapid regulation of graded metabolic output.
-
A face–heart connection evolved as source nuclei of vagal pathways shifted ventrally from the older dorsal motor nucleus to the nucleus ambiguus. This resulted in an anatomical and neurophysiological linkage between neural regulation of the heart via the myelinated vagus and the special visceral efferent pathways that regulate the striated muscles of the face and head, forming an integrated social engagement system (Figure 1; for more details, see Porges7,15).
-
With increased cortical development, the cortex exhibits greater control over the brainstem via direct (eg, corticobulbar) and indirect (eg, corticoreticular) neural pathways originating in motor cortex and terminating in the source nuclei of the myelinated motor nerves emerging from the brainstem (eg, specifi c neural pathways embedded within cranial nerves V, VII, IX, X, and XI), controlling visceromotor structures (ie, heart, bronchi) as well as somatomotor structures (muscles of the face and head).

